Dr. Reshma Ramachandran, winner of the 2025 Bernard Lown Award!
The Bernard Lown Award for Social Responsibility, created in honor of Dr. Lown after his death in 2021, recognizes young clinicians who stand out for their bold leadership in social justice, environmentalism, global peace, or other humanitarian efforts.
The Lown Institute is proud to present Reshma Ramachandran, M.D., M.P.P., M.H.S., with the 2025 Bernard Lown Award for Social Responsibility, for her exceptional work promoting equitable access to medicines, mobilizing physicians nationwide to challenge pharmaceutical industry influence, and advancing legislative reforms that prioritize the public’s health over corporate profit. (press release)
Dr. Ramachandran is the chair of the FDA Task Force for Doctors for America. She is also an assistant professor, board-certified family physician, and co-founder and co-director of the Yale Collaboration for Regulatory Rigor, Integrity, and Transparency. Inspired by the inequitable access of medications during her time with the Medical Research Council in Cape Town, South Africa, Dr. Ramachandran dedicated her career to advocating for improved pharmaceutical policy. Her work has led her to author numerous op-eds and testify before the U.S. Congress multiple times, ultimately leading to reforms within the FDA that have improved drug development and funding.
See the timeline below to learn more about Dr. Ramachandran’s life and accomplishments.
Dr. Ramachandran’s Story
Reshma Ramachandran is born
She is born in Kottayam, Kerala, India.

Moves to the United States
Reshma and her mother move to Columbus, Ohio to join her father, a postdoctoral fellow and scientist at Ohio State University.
Two years later, the family relocates to Miami, Florida, as her father takes on a new role as a cancer researcher at the Sylvester Comprehensive Cancer Center at the University of Miami.

Wins her first academic award
In high school, Reshma wins first place in the Intel International Science and Engineering Fair in Health and Medicine.
She spends countless hours after school in her father’s lab during this time, developing a love of science that motivates her to pursue a career in biomedical research.

Her professional trajectory shifts
While pursuing a Sc.B. in Physics at Brown University, including thesis work on “lab-on-a-chip” technologies, she has the opportunity to work as a research assistant with the South African Medical Research Council.
Here, after witnessing the research and advocacy of clinicians and public health professionals, she comes to understand that the development of new health technologies alone will not ensure patient access to better health. This realization shifts her trajectory toward health policy.

Earns undergraduate degree and begins medical school
She graduates from Brown University with a Sc.B. in Physics and matriculates to the Alpert School of Medicine at Brown University.
The summer before medical school, she interns with the American Medical Student Association in Washington, DC during a pivotal moment in the national debate over healthcare reform. Her eyes are opened to the role of other actors outside of patients and clinicians in influencing health policy–namely that of the pharmaceutical, medical device, and insurance industries.
Begins Global Health Fellows Program
Reshma is accepted into the Global Health Fellows Program with the Duke University Sanford School of Public Policy directed by Dr. Anthony D. So. As part of this fellowship, she
interns at the World Health Organization’s Patient Safety Programme, allowing her to make site visits across UN agencies, intergovernmental organizations, and global non-profit organizations. Dr. So opens her eyes to the global, complex landscape of the access to medicines movement and becomes her longtime mentor and colleague.

Serves as PharmFree Fellow with AMSA
Dr. Ramachandran takes time off from medical school to serve as the inaugural Pharmaceutical Policy (PharmFree) Fellow with the American Medical Student Association, where she works with academic medical centers to strengthen their financial conflict-of-interest policies with the pharmaceutical and medical device industries.
She also leads national efforts among medical student associations during the ongoing negotiations for the Trans-Pacific Partnership Agreement, advocating against price gouging and the dismantling of public health protections through trade policy.
She also joins the National Physician Alliance, where she becomes the Co-Chair of the FDA Task Force.
Graduates with M.D. and M.P.P.
Dr. Ramachandran graduates from both the Alpert Medical School at Brown University and the Harvard Kennedy School with an M.P.P. with a focus on International Trade and Finance.
She takes a detour in her medical career, joining Dr. Anthony D. So as he launches the “Innovation+Design Enabling Access” (IDEA) Initiative at the Johns Hopkins Bloomberg School of Public Health as a research faculty member. She gains intensive experience in domestic and global health policy, with her work spanning U.S. drug pricing legislation and global efforts to combat antimicrobial resistance.
She joins Doctors for America, where she eventually re-establishes and chairs the FDA Task Force following its 2019 merger with the National Physician Alliance.

Joins UAEM as board member
Dr. Ramachandran becomes a board member of the non-profit organization, Universities Allied for Essential Medicines (UAEM) North America. UAEM is a global student organization advocating for affordable access to technologies discovered and developed on university campuses. By 2022, she will become the president of the Board of Directors.

Begins medical residency in California and continues her activism
She begins her family medicine residency at Kaiser Permanente Los Angeles Medical Center and University of California, Los Angeles, where she also teaches medical trainees about pharmaceutical industry conflicts of interest and prescription drug pricing policy.
In California, she continues her activism by partnering with Universities Allied for Essential Medicines at UCLA to push for global affordable access to a prostate cancer drug discovered and developed on campus. Her efforts are ultimately successful and the university adopts a policy ensuring access to all university-owned health technologies.

Speaks at Lown Institute’s 2018 conference
In April, Dr. Ramachandran is a speaker at the Lown Institute’s 2018 conference, where she speaks about state and national efforts to lower high drug prices.
Later in the year, she co-authors an investigation finding that many drugs granted accelerated approval lack evidence of clinical benefit despite accounting for substantial CMS spending.

Begins postdoctoral fellowship at Yale
She wins both the Association of Family Medicine Residency Directors Resident Award for Advocacy and the Society of Teachers of Family Medicine Resident Teacher Award. She also co-authors a book chapter with Dr. Anthony D. So on the role of civil society in tackling antimicrobial resistance.
She moves to New Haven, CT with her husband and infant to start a postdoctoral fellowship with the National Clinician Scholars Program at Yale.
Testifies before the U.S. House Ways and Means Committee
She wins the Doctors for America Founders Award for Excellence in Advocacy.
In February, she testifies before the Health Subcommittee of U.S. House Ways and Means about COVID-19 immunizations, discussing her research on the pricing of influenza vaccines.
She publishes a piece in JAMA on an investigation that found shortcomings by the FDA and NIH when it comes to clinical trial results reporting as required under law.

Co-founds the Yale CRRIT and testifies before the U.S. House Energy and Commerce Committee
After completing her postdoctoral fellowship with the National Clinicians Scholars Program, Dr. Ramachandran joins the Yale University School of Medicine as an Assistant Professor in the Section of General Internal Medicine.
She also begins working as a primary care physician at the Cornell Scott Hill Health Center, a federally qualified health center in New Haven, CT.
In her new faculty role, she co-founds the Yale Collaboration for Regulatory Rigor, Integrity, and Transparency (CRRIT) with her co-director and mentor, Dr. Joseph S. Ross. CRRIT brings together multidisciplinary faculty to research medical product evaluation, approval, and coverage aimed at advancing policies to improve patient health. She testifies multiple times before the U.S. House of Representatives Energy and Commerce Committee on FDA user fee program reauthorization, advocating for reforms to enhance regulatory rigor and transparency.

Speaks as expert witness for U.S. Senate HELP Committee
Dr. Ramachandran speaks as an expert witness before the U.S. Senate Health, Education, Labor, and Pensions Committee in a session titled “Preparing for the Next Public Health Emergency: Reauthorizing the Pandemic and All-Hazards Preparedness Act.” She discusses her research on clinical trial evidence and the regulatory and financial incentives for pandemic treatments and vaccines.
She and Dr. Ross, represented by Democracy Forward, file an amicus brief on the Relentless v Department of Commerce case (later enjoined with Loper Bright v Raimondo), addressing the impact of potentially overturning the longstanding Chevron doctrine on FDA and medical product regulation. She and her co-authors later write about these implications in JAMA.
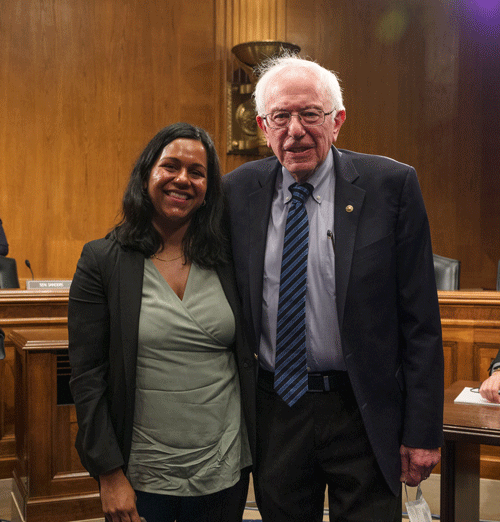
Speaks out about mifepristone access and the affordability of GLP-1 medications
Through her work with Doctors for America, Dr. Ramachandran helps to shape an amicus brief in Alliance for Hippocratic Medicine v FDA in support of the FDA’s continued authorization of mifepristone, one of the two pills used in medication abortion.
She and CRRIT postdoctoral fellow Dr. Melissa Barber, along with other health advocates, are invited to be part of an expert roundtable hosted by Senator Sanders on the “Outrageous Cost of Ozempic and Wegovy in America.”

Wins Bernard Lown Award for Social Responsibility
Dr. Reshma Ramachandran wins the 2025 Bernard Lown Award for Social Responsibility. She is recognized for her exceptional work promoting equitable access to medicines, mobilizing physicians nationwide to challenge pharmaceutical industry influence, and advancing legislative reforms that prioritize the public’s health over corporate profit.

