Will it take 50-100 years to get the right answer about tPA for stroke?
By W. Ken Milne and Daniel Fatovich
It was the neurologist Dr. John Hughlings Jackson who said “It takes 50 years to get a wrong idea out of medicine, and 100 years a right one into medicine.” Ironically, a common neurological treatment is one of those ideas that many emergency physicians consider to be wrong while most neurologists consider it to be right, no matter how much research is done.
We all want patients with a stroke to get the right care, at the right time with the right treatment, guided by the best available evidence. Whether or not using thrombolysis, treatment that tries to dissolve blood clots, improves outcomes for patients with acute ischemic stroke has been arguably the most controversial debate in emergency medicine and neurology over the last few decades. Theoretically, drugs like tissue plasma activator, or tPA, can dissolve the blood clots that cause some strokes. However, these drugs can also cause bleeding in the brain, a serious and sometimes fatal side effect.
The question is, do the potential benefits of tPA outweigh the potential harm of brain hemorrhage? We examine this issue in light of new evidence.
What do the randomized trials find?
There have been twelve randomized controlled trials (RCTs) of thrombolysis for acute ischemic stroke between 1995 and 2012. (See the table below for a summary of the results from these studies). These led to different treatment recommendations by American College of Emergency Physicians (ACEP) and American Heart Association/American Stroke Association (AHA/ASA) for thrombolysis using tPA, up to 4.5 hours after stroke onset.
This list does not include two newer trials (one from 2019 and another from 2016) looking at extending the therapeutic window to 4.5 to 9 hours. These newer trials were done with advanced brain imaging (CT perfusion or perfusion-diffusion MRI) selecting patients with a perfusion mismatch. Both trials were stopped early which can introduce bias towards efficacy. In addition, the majority of patients included in the two RCTs extending the time window would now qualify for endovascular therapy (EVT) clot retrieval. EVT has more evidence for efficacy than systemic thrombolysis. A recent trial has even shown that EVT alone is non-inferior to EVT plus tPA .
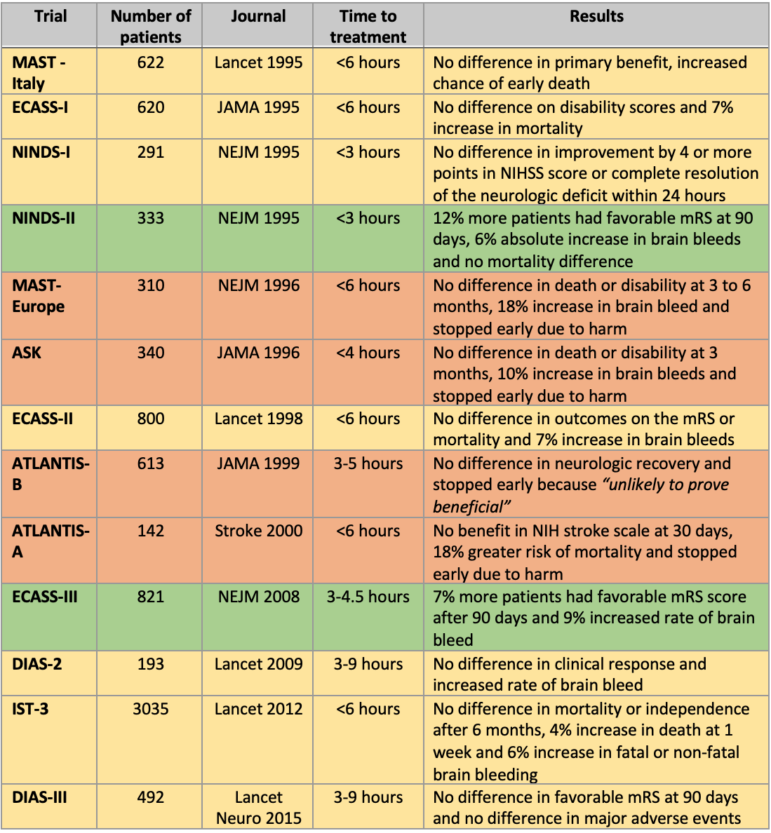
Of the twelve thrombolysis RCTs, only two claimed benefit (NINDS-2 and ECASS-3), and four were stopped early for harm (bleeding) or futility. The other eight RCTs failed to demonstrate efficacy of these drugs. Let’s look a little closer at some of the key trials for tPA, which is currently the only FDA-approved thrombolytic for acute ischemic stroke.
Trials that failed to show benefit of tPA:
ECASS-I randomized patients to receive 1.1mg/kg of tPA or placebo up to six hours. The results favored tPA at 90 days. However, there were more bleeds in the tPA group, no statistical difference in mortality at 30 days, but more deaths at 90 days in the tPA group. The conclusions were the potential benefit of treatment did not outweigh the harms.
NINDS -1 randomized patients to receive 0.9mg/kg of tPA up to three hours after onset of symptoms. There was no statistical difference in their primary outcome of improvement of four points over base-line values in NIHSS (National Institutes of Health Stroke Severity score) or resolution of the neurologic deficit within 24 hours of the stroke onset.
ECASS-2 randomized stroke patients to receive 0.9 mg/kg tPA up to six hours. There was not a statistical difference in the modified Rankin Scale (mRS) score at three months. There was increase in bleeding but no difference in mortality at 30 or 90 days between groups. Notably, the subgroup analysis of patients in the less than 3-hour treatment window did not confirm the benefit of tPA reported in NINDS-2.
IST-3 was the largest RCT, randomizing 3,035 patients to 0.9mg/kg of tPA or placebo up to six hours. They found no statistical difference in alive/independent of activities of daily living at six months. There was a 4% absolute increase in death within seven days with tPA and a 6% increase in fatal or non-fatal intracranial hemorrhage with tPA. This means that for every 25 patients treated with tPA there would be one additional death in the first week. There were many problems with IST-3 that have been described in post-publication review, including the lack of blinding and selection bias.
It is important to note that IST-3 had a 3 and 4.5 hour subgroup of 1,177 patients. This represents almost a 50% greater number of patients than were included in ECASS-3 (n=821) claiming benefit with tPA. In this pre-specified subgroup analysis, there was a 6% absolute worse outcome with tPA. Good neurologic outcome was 38% in the placebo group and only 32% in patients randomized to tPA. This 6% difference is statistically significant using the 95% confidence intervals.
Trials that claim to show benefit of tPA
NINDS -2 is one of the two RCTs claiming to demonstrate superiority of tPA over placebo in stroke patients. It randomized 333 patients to receive 0.9mg/kg of tPA up to three hours after stroke symptoms. There was a 12% absolute benefit at 90 days, 6% absolute harm (intracerebral hemorrhage) and no statistical difference in mortality at 90 days.
If tPA really works, the trial would show that there was a bigger improvement in outcomes with tPA, compared to the starting point.
The problems/limitations with NINDS have been pointed out in post-publication review. A reanalysis of the NINDS data by Dr. Hoffman and Dr. Schriger was published in Annals of Emergency Medicine in 2009. The bottom line from this reanalysis was that the baseline imbalance in stroke severity led to the difference in outcomes. After adjusting for the baseline imbalance, tPA failed to show superiority. A neurologist, Dr. Saver, and colleagues have questioned this reanalysis of NINDS.
Why is it important to adjust for patients’ severity of the stroke? People who start out with a more severe stroke will typically have a worse outcome than people who started with a mild stroke. So, if tPA really works, the trial would show that there was a bigger improvement in outcomes with tPA, compared to the starting point (baseline stroke severity). However, in the NINDS-2 trial, there were more people with severe strokes in the placebo group and more mild strokes in the tPA group. That is why the results made it look like tPA was more effective than placebo, but the reanalysis shows that the two groups of patients simply had a different starting point. When you take the starting point into account, there was no difference between tPA and placebo, but a lot more instances of harm with tPA.
What does the new research tell us?
ECASS-3 is the only other of the two RCTs claiming to demonstrate superiority of tPA. This trial randomized patients to receive 0.9mg/kg of tPA between 3 and 4.5 hours after onset of symptoms or placebo. ECASS-3 reported a 7% absolute benefit of improved mRS score at 90 days compared to placebo, 9% increase in intracranial hemorrhage, 2% increase in symptomatic intracranial hemorrhage and no significant difference in mortality.
A recently published reanalysis of ECASS-3 by Dr. Brian Scott Alper and other researchers at EBSCO Health and McMaster University calls into question the efficacy of tPA between 3 and 4.5 hours after onset of stroke symptoms.
In their reanalysis, the authors adjusted for baseline imbalances in stroke severity. Using multiple approaches, the authors were unable to demonstrate any significant benefit to tPA while reconfirming the harm. They concluded by calling upon clinicians, patients and policymakers to re-consider using tPA in patients with acute ischemic strokes.
This study is similar to the reanalysis of NINDS-2: when you take into account the starting point of how severe the stroke was when they received the tPA or placebo, there was no difference in outcome. In addition, in ECASS-3 there were significantly more people in the placebo group who had had a previous stroke, than in the tPA group. Typically, when someone has had a previous stroke and then has a recurrence, they will not do as well as someone with a first-time stroke. The whole trial result can be explained by these differences between the groups. When Alper et al. took these baseline differences into account in their reanalysis, they found no evidence for using tPA.
Examining conflicts of interest
It is known that financial conflicts of interest (fCOI) can influence studies and introduce bias into research. This can be also be seen when studies are analyzed in a Systematic Review/Meta Analysis (SRMA), which is when researchers analyze a body of already-published research on a subject. Publications with fCOI tend to have more positive conclusions and are of lower methodological quality compared to those without fCOI, according to a Cochrane Review. In addition, journalist Jeanne Lenzer has published in the BMJ why we can’t trust the clinical guidelines on this issue of tPA for acute ischemic stroke.
Looking at two SRMAs of the research, one can see how fCOI may impact the results. One review by Emberson et al. found that tPA “significantly improves the overall odds of a good stroke outcome when delivered within 4.5 h of stroke onset,” but the authors only included nine of the available RCTs. When you look at the disclosures reported in the study by Emberson et al., there are multiple conflicts reported including: research grants, advisory board membership, honoraria, speaker fees, travel support from Boehringer Ingelheim who markets and sells tPA. In contrast, a review from Donaldson et al., which looked at 26 RCTs of thrombolytics and found no benefit, the authors report no competing interests.
To summarize, there are no RCTs in patients not qualifying for EVT that show benefit of tPA for acute ischemic stroke now that NINDS-2 and ECASS-3 have been reanalyzed. We agree with Alper et al., Donaldson et al., Hoffman and Cooper, and others that have questioned this treatment modality and called upon it to be re-evaluated. Hopefully this won’t take 50 years to 100 years to get the right answer.
W. Ken Milne MSc, MD, CCFP-EM is Chief of Staff at the South Huron Hospital Association and Adjunct Professor at the Schulich School of Medicine and Dentistry.
Daniel M. Fatovich, MBBS, FACEM, PhD is an Emergency Physician and Director of Research at Royal Perth Hospital; Head of the Centre for Clinical Research in Emergency Medicine at the Harry Perkins Institute of Medical Research; and Professor of Emergency Medicine at the University of Western Australia.
